Summary about SUGAM Portal:
- The Central Drug Standard Control Organisation (CDSCO) is the national and official regulatory authority in India.
- On the 14th of November 2015, CDSCO launched an online e-Governance portal called SUGAM Portal for performing various CDSCO duties related to permissions and approvals of drugs and different entities.
- The distinct functions carried out in SUGAM Online Portal are applying for NOCs, licenses, permissions, approvals, and registration certificates etc.
- The SUGAM online e-Governance portal requires registration of the applicant. The applicant must create their profile and submit hard copies of all the mandatory documents. After their application is submitted, it is evaluated by the CDSCO, and it is either approved or rejected. Only after CDSCO’s approval, the applicant can login to SUGAM Portal.
- The CDSCO allows many types of entities and a wide range of applications to be filed in SUGAM Online Portal.
- The CDSCO also requires a comprehensive list of mandatory documents for registration and licensing processes.
- In conclusion, SUGAM has made it easier for manufacturers, importers, and businesses in India to have an interface for all their licensing and regulatory needs.
What is SUGAM Portal?
As digitization of Indian initiatives is on the rise, the CDSCO has also joined in this movement and launched an online e-Governance portal called SUGAM. The purpose of SUGAM is to facilitate drug licensing processes such as permissions, approvals, application tracking, registration certificate etc.
SUGAM has a single easy user interface which simplifies all the complex processes regarding drug licensing for all businesses and manufacturers. It keeps the entire licensing process from pre-registration to grant of approval, seamless and accountable.
Central Drug Standard Control Organisation (CDSCO)
Under the Drugs and Cosmetics Act, 1940, any substance which is defined as a drug (under Section 3b of Drugs and Cosmetics Act 1940) must be registered before its import to India. The sales, distribution, manufacturing and import of drugs is regulated under the Drugs and Cosmetics Act 1940. The manufacturing site must also be registered for import purposes.
The CDSCO is the national authority of India which regulates the above Act. Along with approvals of drug licensing, they also provide drug standards which must be followed, provide expert advice to state drug control organisations, and perform quality checks of imported drugs.
Features Of CDSCO SUGAM Online Portal
SUGAM is the online e-Governance portal which was launched by the CDSCO on the 14th of November 2015 to address its drug licensing permissions and approvals across India. It is a seamless interface whose functions include:
- The CDSCO has updated educational information on licensing drug manufacturing facilities, licensed drug formulations and drugs manufactured in India.
- It has a complete database of all the permissions and licenses for manufacturers, manufacturing sites and drug formulations granted by state drug control organizations.
- Applications for NOCs, permissions, approvals, licenses, and registration certificates.
- Applicants can keep track of their applications, respond to queries by CDSCO and download permissions issued by the CDSCO.
- Applicants can keep track of their saved, submitted, approved, rejected and ongoing licensing applications.
- It consists of a notification announcement section which helps in keeping track of latest updates in rules.
- SUGAM also allows CDSCO officials in online processing of applications, generating permissions and MIS reports.
| Central Drug Standard Control Organisation (CDSCO) | |
| Central Authority | State Authority |
| Approval of new drugs, registration certificate, Import license, Cosmetic import registration certificate | Drug license for sale, distribution and manufacture of new drugs |
| Laying down the standards for drugs | Drug testing laboratories |
| Control on quality of imported drugs | Pre and post licensing inspections |
Table 1: Authorities under the Drugs and Cosmetics Act, 1940 and Drugs and Cosmetics Rule, 1945
Pre – Registration process in SUGAM Online e-Governance Portal
The SUGAM online e-Governance portal is launched to access online CDSCO services for all manufacturers and businesses across India. The following are the steps required for applicants to register in SUGAM:
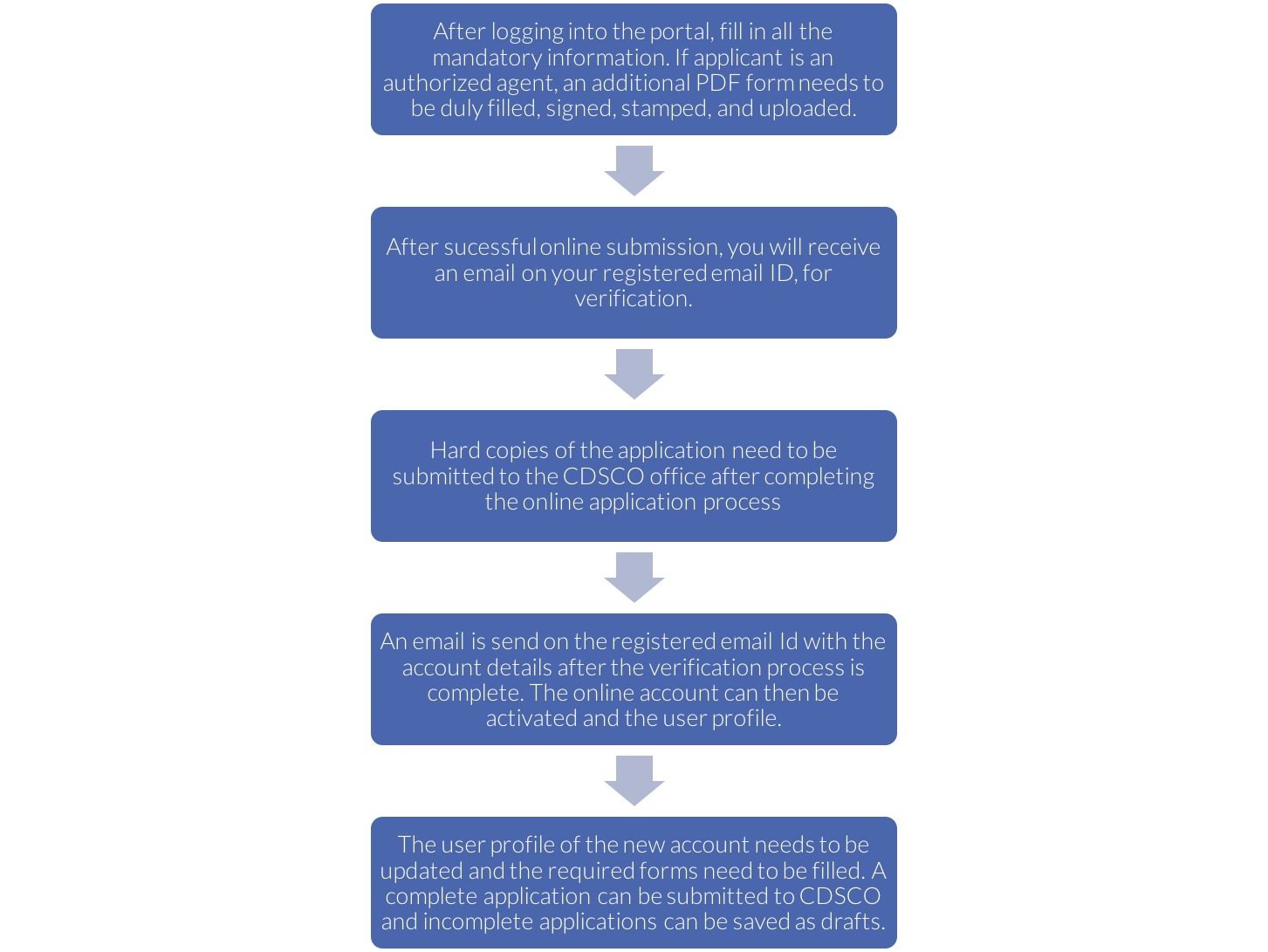
- The authorised signatory/applicant must fill the registration form and submit it along with hard copies of all the mandatory documents such as address proofs, ID proofs, undertakings etc (to the CDSCO office).
- The CDSCO officials will then evaluate the application and documents submitted and grant their approval or rejection. If the application has been approved, the registration will be successful, and the applicant can now login to SUGAM.
- However, if the application is rejected, an email will be sent to the applicant by the CDSCO officials stating the reason of rejection.
Types of SUGAM Applications
The CDSCO has granted many types of applications to be filed in SUGAM. They are:
- Certificate of Registration – Form 41 for Drugs
- Certificate of Registration – Form COS-2 for Cosmetics
- License of Import – Form 10 for Drugs
- NOC for a clinical trial for import/manufacturing of a new drug (Form CT-06/Form CT-19/ Form CT-20/ Form CT-22/ Form CT-23
- Clinical trial test license
Types of SUGAM Entities
The following entities can successfully register in SUGAM:
- Indian agents
- Corporates
- Foreign enterprises with Indian subsidiaries
- Importers
- Cosmetics
- Ethics Committee
- R & D organisations
SUGAM – Sub-Login accounts:
CDSCO has also given the provision for corporate applicants to set up Sub-Login accounts for their:
- CROs
- Branch offices
- R & D sites
- Manufacturing sites
Conclusion
In conclusion, SUGAM offers a remarkably simple and user-friendly single interface which provides transparency in all steps of the licensing process. This type of interface also creates accountability amongst all stakeholders involved in a manufacturing business as all of them can view the status of their application.
