Table of Contents
ToggleMedical Device: Manufacturing License For Medical Devices
The Central Drugs Standard Control Organization (CDSCO) is the country’s regulator for Indian medical devices and pharmaceuticals and has a parallel role in conjunction with FDA. Food and Drug Administration of the United States (FDA) It is the goal of the government that will bring medical equipment, which includes contraceptives and implants into the view by the Central Drugs and Standard Control Organization (CDSCO).
CDSCO manufacturing licenses for medical devices falls under the CLAA scheme. The agreement approved the license by State Licensing Authority may be examined to be approved by CLAA under the condition that the applicant comply with the conditions that are stipulated in the Medical Devices Rules. Manufacturing of medical devices notified within the CLAA scheme to be sold in India. Manufacturing license form -28 required under the Drug & cosmetic rules for the granting of a manufacturing license. Recently, CDSCO issued a new notice of the requirement to register for medical devices Classes A and B.
Medical Devices Classes’ Classification
The first and most important thing to do is to determine the classification of your medical device since the process for applying and obtaining a license may differ in accordance with the classification that the gadget is. MDR 2017 has categorizes devices into Class A, Class B and Class C. D. Below table will shed some clarity on the how medical devices are classified. If you are planning to produce medical devices, you must sign up with the CDSCO Sugam Online Registration portal first.
| Type | Risk | Example |
|---|---|---|
| Class A Medical Device | Low risk | Bandage, examination loves etc. |
| Class B Medical Device | Low-Medium Risk | B.P. monitoring device, thermometer |
| Class C Medical Device | Moderate to High Risk | Implants, catheter |
| Class D Medical Device | High Risk | Heart Valve |
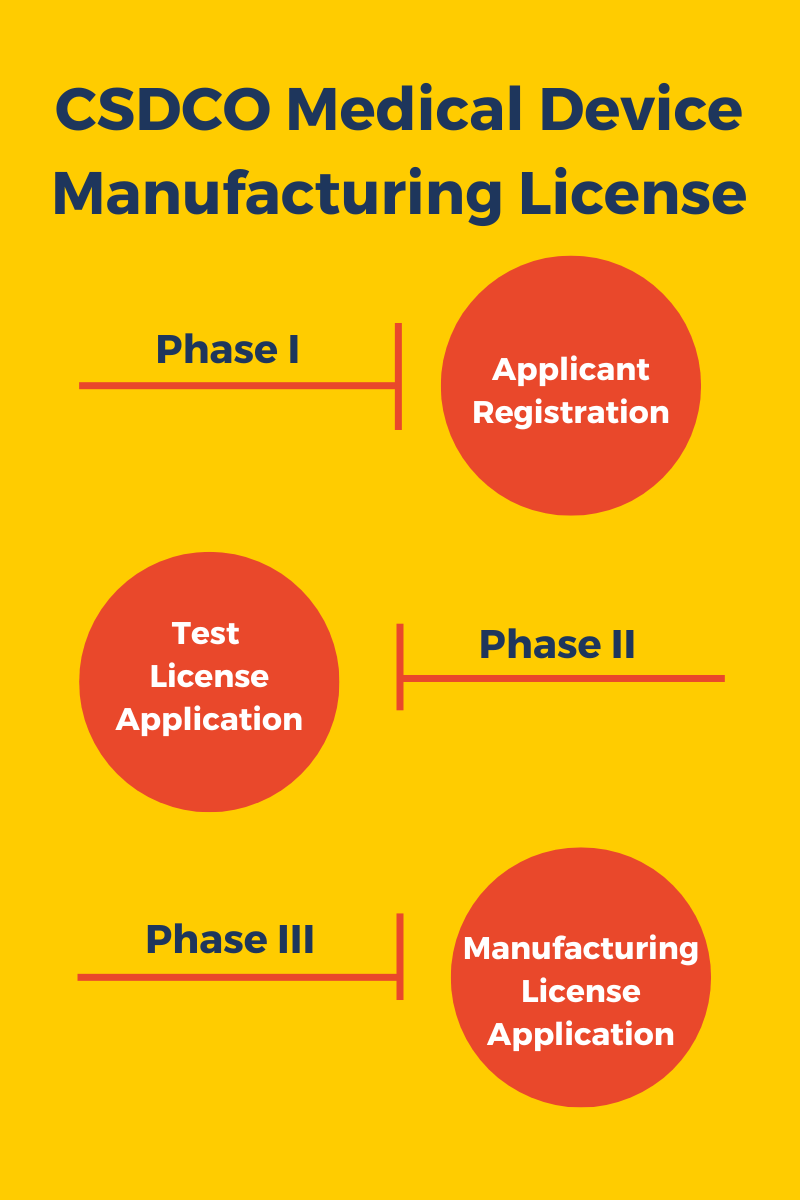
Phases of Medical Device Manufacturing License Approval
- Phase I – Applicant Registration
- Phase II – Test License application
- Phase III – Manufacturing license application
Pre-requisite Of Manufacturing Medical Device
- Generic Name / Brand Name
- Intended Use
- Material of construction
- Mode of application
Phase I – Site Applicant Registration - Get Permission To Manufacture Medical Device Site
In this process, the clients can be registered with an active account on the CDSCO Online Portal for Medical Devices registration site.
The requirements for documents are the same for medical Device Manufacturing Application Registration
The documents required for this stage are address pro or similar certificates of registration or certificates of incorporation or MTNL/BSNL bill of corporate website and ID proof of an authorized person. This person is any individual aside from the management team, who can be authorized for all kinds of registration.
Procedure For Manufacturing License Of Medical Device Application Registration
• CDSCO Online form submission.
• Uploading company and document details and documents.
After submission of the form, a preliminary approval mail will be sent from CDSCO after which another confirmation mail with the date of the hard copy submission has been received.
Phase II – CSDCO Manufacturing Test License For Medical Devices
This medical device manufacturing test license is necessary to be able to produce a small-sized devices to test, evaluate demonstration, and training of employees.
• Procedure For Manufacturing Medical Device Test License
• Apply online through a portal
• Form MD-12 must be filled out with accurate information
• Uploading documents.
• Fees payment.
• Status change for the application
Phase III – CDSCO Manufacturing License Application For Medical Devices
The application for a medical device manufacturing license is required to get the approval to make medical devices for commercial use.
Procedure For Medical Device Manufacturing License Application
• Online application submission
• In order to manufacture Class Aand Class B devices, MD-3 Form must be filled in with the correct information.
• Uploading documents.
• Fees are paid in accordance with CDSCO
• Receiving a letter about the refusal or approval of a license.
Note that any substance which falls into the definition of a substance (Section 3b in the Act) is required to be registered prior to manufacture.
How Can CliniExperts Help In CDSCO Manufacturing License?
To be able to apply for manufacturing permits, you have to make the plant master file as well as technical documents for submissions. This is a crucial and lengthy process for manufacturers and for over 10 years, we have been helping medical device makers in creating plant master files and offer DMF submission guidelines for manufacturers. To register for CDSCO on-line registration, or for information about how to get a CDSCO manufacturing license for your device, please contact CliniExperts today to discuss your requirements. CliniExperts medical device consultancy offers services across the world. Get a CDSCO registration license and get best service.
